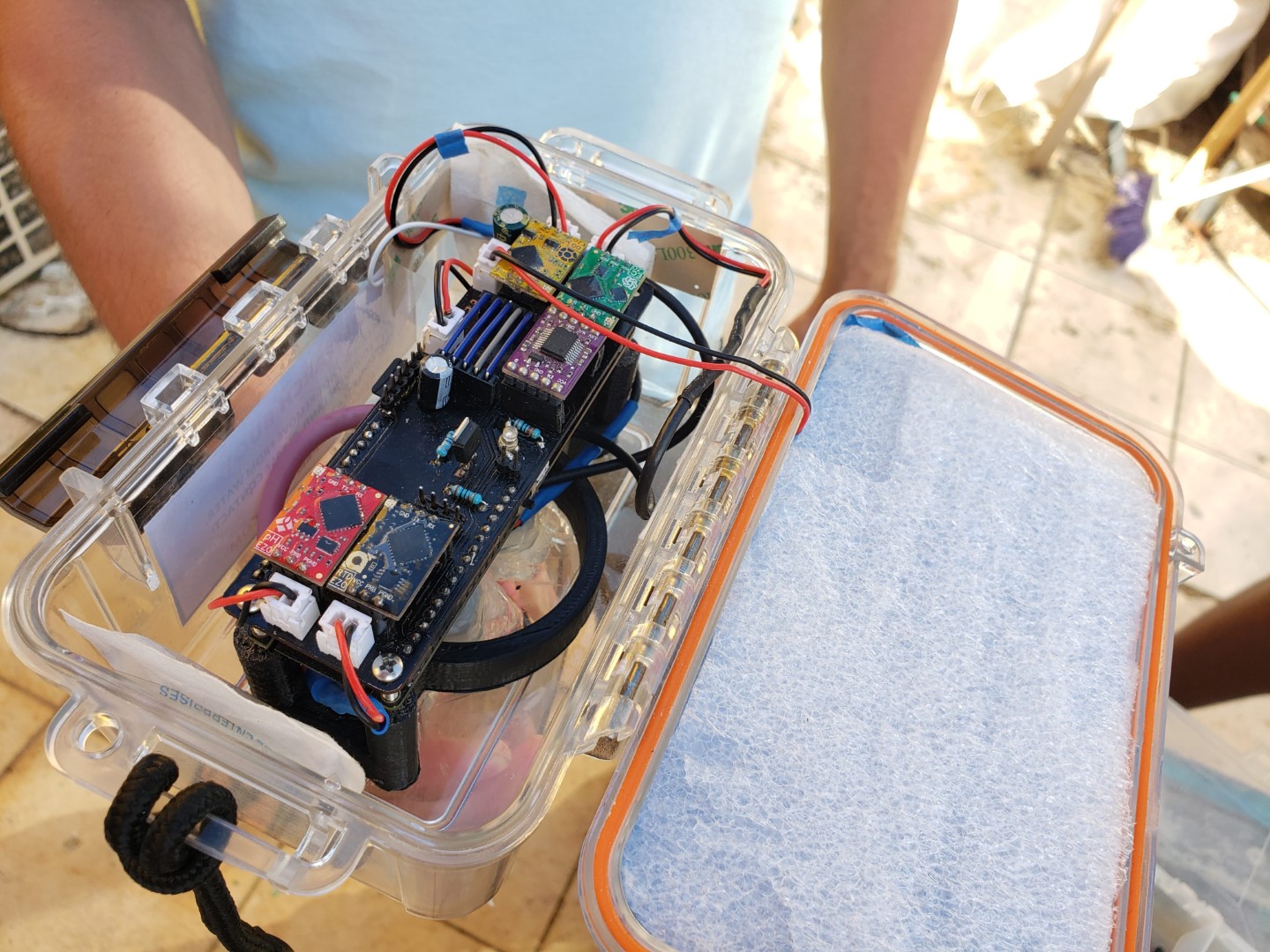
The water quality monitor collects and reports measurements from sensors suspended in the ocean. Currently we are upgrading our prototype and plan to have it back at Surfside in a week or so.
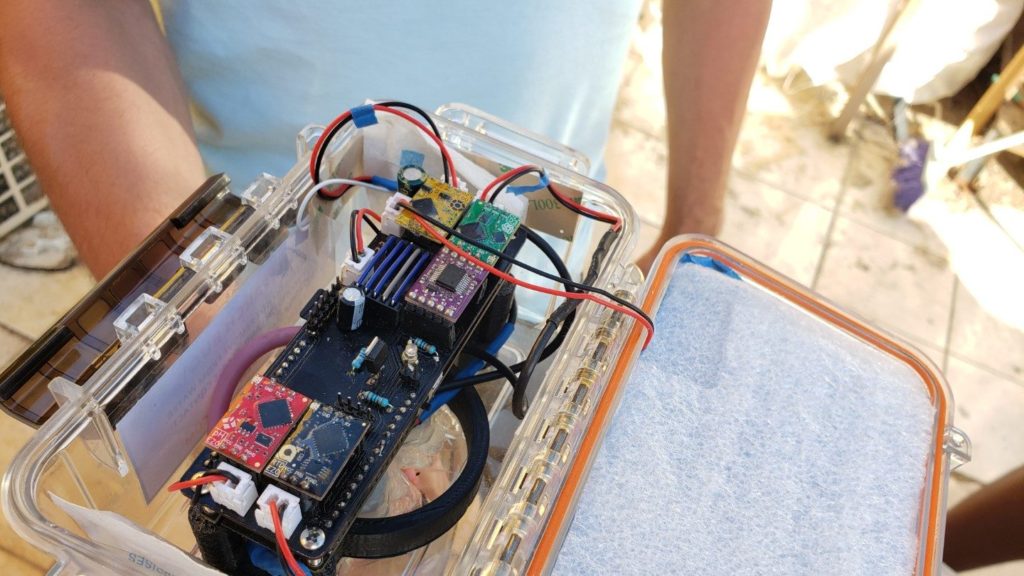
Expanding on our previous water quality experiments, this effort seeks to develop a system for continuous monitoring of aquatic environmental parameters that can be installed off grid, powered by solar panels and communicating through GSM networks. Once operational, this system will be tested against commercial water quality meters and standardized monitoring methods to validate its accuracy.
During a two-month coding extravaganza, Manuel and Sean built the SurfsideSensors repository and prototype environmental monitoring stations for water and air quality. The stations use the same code, with different libraries and deployments for different sensors.
The water quality station, named WAP (water assessing probes), makes use of affordable and high precision probes manufactured by Atlas Scientific. For this station, the included probes measure temperature, pH, electrical conductivity, and dissolved oxygen. Each probe uses a signal interpreting communication circuit that takes the raw signal from the probe, and sends it to the microcontroller on request. The microcontroller that was used includes an onboard battery holder, solar panel port, gps receiver, SD card slot, and a GSM modem with SIM card. This avoids having to find and buy all of these components separately and reduces risk of wiring something up wrong. The enclosure was inspired by the design of MakerBuoy, which uses a watertight utility box and a bottom tube to keep it upright and protect the probes.
The initial development focused on software, followed by construction of a custom circuit board to mount the components. The enclosure was produced last, and the prototype was calibrated and installed on August 22 at Surfside Marina. The unit worked as expected except for the readings of the pH sensor, which fluctuated a lot and reported readings that didn’t make sense for ocean water. After two weeks the unit was removed from the water and inspected. Attempts to recalibrate didn’t work as expected, so we thought that the sensor might have been damaged or defective. Biofouling was observed on the device, which was a concern from the start. Awaiting a replacement pH sensor and restocking of calibration fluids, WAPv1 is currently back at the lab for servicing.
Coming Soon Manual to build water quality sensor
Coming soon link to DATA
What is dissolved oxygen?
Like in the air, there is also oxygen in the water, but it is dissolved. Just like salt mixes with water, oxygen dissolves in water. This is an important aspect to measure because the level of dissolved oxygen affects the life underwater. Every plant, fish, and other organisms underwater need oxygen to survive, but the only way to obtain oxygen is through dissolved oxygen.
Where does oxygen come from?
There are two main ways that contribute to dissolved oxygen. All substances always want to move from a high concentration to a lower one, and you will find that oxygen is transferred and absorbed by the ocean from the air. You can imagine it like being in a crowded room, everyone piled on top of each other, and there is another room next door where there is more space. Naturally, people will try to move to the room with more space. This is how oxygen in the air and water works. Therefore, oxygen from the air enters the water because there is more space for it.
In addition to absorption from the air, oxygen also comes from photosynthesis. Plants capture and use the sunlight and carbon dioxide from the air to produce, among other things, oxygen. Not only terrestrial plants but also aquatic organisms can produce food from photosynthesis, such as aquatic vegetation, seaweed, and phytoplankton (also known as microalgae), which includes cyanobacteria and algae. These organisms are essential for oxygen production on our planet. In the process of photosynthesis, they create oxygen that dissolves directly in water. It is important to note that the exchange of oxygen between the ocean and the air occurs constantly, so not only does the ocean absorb oxygen from the air, but it also sends oxygen back to the air, especially because of the high production of oxygen from photosynthesis.
What happens to the oxygen in the water?
Just like on land, everything that lives in water needs oxygen to survive. Even when oxygen leaves the water and goes into the air, there is always a certain amount of dissolved oxygen remaining in the water. However, the amount of dissolved oxygen depends not only on the amount of oxygen in the air but also on the amount of salt and the temperature of the water. Salt also occupies space in water, so it competes with oxygen for space. Therefore, oxygen becomes much scarcer when there is a lot of salt in the water. Another factor that can affect dissolved oxygen is temperature. When the temperature causes the oxygen molecules to vibrate and move around a lot, it also makes it easier for oxygen to escape from the water.
Why should we be concerned about dissolved oxygen in our oceans?
Because everything that lives on or in the water needs oxygen to survive. Therefore, scientists measure dissolved oxygen levels to determine the health of the ocean. Fish and other organisms living in water have different oxygen requirements, and they live in different areas of the water. For example, salmon needs a lot of dissolved oxygen, so they live in areas where the water temperature is colder. However, guppies don’t need as much dissolved oxygen, so they can live in warmer water.
Apart from all of this, there is a process called eutrophication. This occurs naturally, but today it happens a lot due to human activities. Eutrophication means an excess of nutrients (such as nitrogen and phosphorus) entering the water, which causes excessive growth of cyanobacteria and algae. You may wonder why this is bad because they produce dissolved oxygen, right? Well, like everything in nature, it all depends on balance. If there are too many algae, they compete with aquatic vegetation by blocking the sunlight. This causes the water to become darker, killing the vegetation, and the algae surpass their own limitations and die. When plants and algae die in large quantities, they undergo decomposition, which consumes a lot of the dissolved oxygen, leaving not enough oxygen for the fish. Finally, fish and other marine life die, causing even more decomposition and depleting almost all of the dissolved oxygen in the water.